Testing in a
T-maze
The T-maze task is an investigation of spatial learning and memory. Subjects are often taught to discriminate between the two arms based on visual, olfactory, tactile or even auditory cues during consecutive trials. Subsequently, reversal learning or retention can be investigated. Alternatively the T-maze is sometimes used for place preference testing. The T-maze is similar to the Y-maze.
High quality mazes
The Noldus T-maze is specifically designed for working with rats or mice. Our standard design is made from grey non-reflective material, and is ideal for video tracking with EthoVision XT and is easy to clean. It also comes with three partitions so you can easily section off each arm.
We also offer a specific T-maze (cross maze) for zebrafish studies.
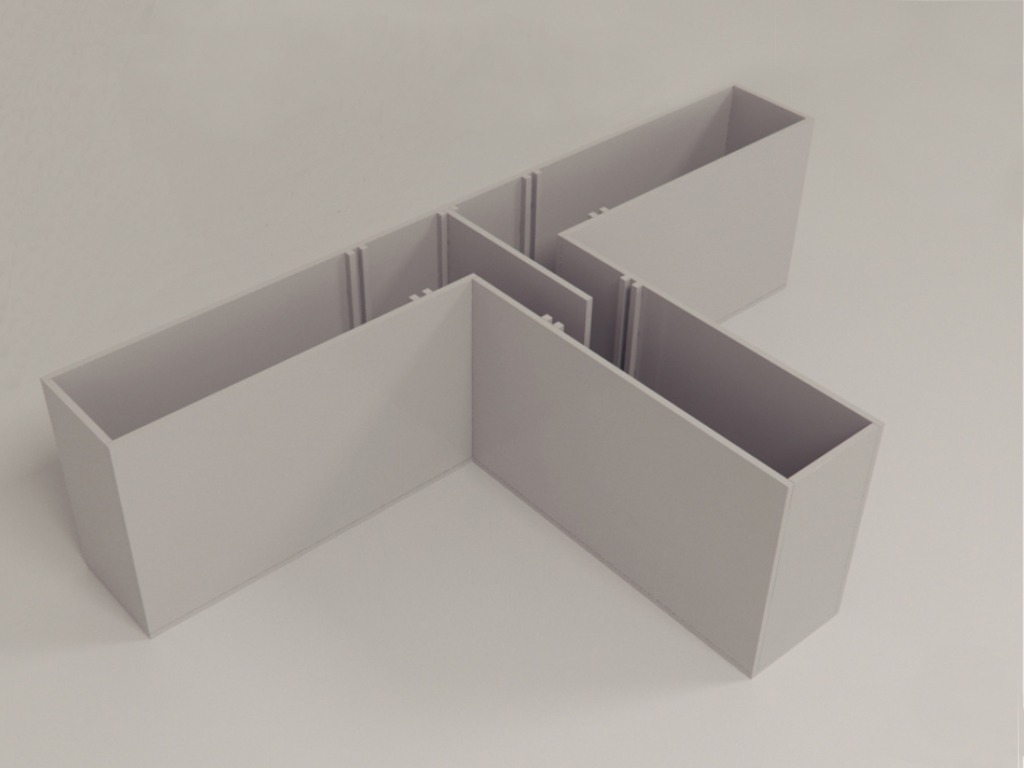
Made for you
The maze can be easily adjusted or built to your specifications. For example, you can choose a different color, a longer start arm, or add an IR backlight. All mazes are available in a cost-efficient package deal including a computer and a full EthoVision XT software license for video tracking. This license can also be used for the video tracking and automation of other behavioral tests.
Free e-book
Basic behavioral neuroscience in rodents
Want to test working memory in rodents? Or short term (spatial) memory in general. In our free basic behavioral neuroscience e-book we explain how these types of memory can be tested in a T-maze. Why and how do we effectively use the T-maze in rodents?
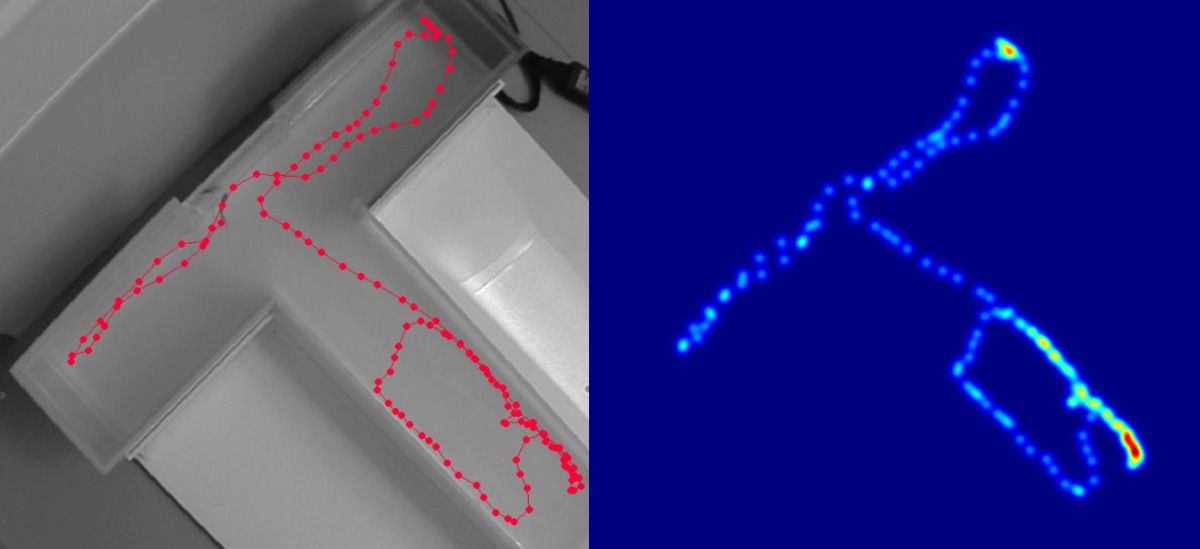
Video tracking
Performing your T-maze test with EthoVision XT video tracking allows you to easily get parameters like latency to enter the correct arm, number of transitions into each arm, time spent in each arm, and more. Using all three body points (nose, center, and tail base), you can acquire very accurate data.
Alternative suppliers
Noldus mazes can be standard or custom build to your specifications, and are optimally suited for video tracking with EthoVision XT, and available in a package deal. Alternatively, we also offer mazes from several suppliers such as Ugo Basile and Maze Engineers (availability may depend on your location).
Relevant blogs

The effect of environmental levels of lead on zebrafish development
Lead exposure has a negative influence on the developing brain and body. Zebrafish research allows us to understand the effects of lead poisoning on the different stages of life.
What we can learn from zebrafish in a T-maze
Scrolling through our recent blogs, you can tell how important zebrafish have become in behavioral research. So we thought it was time to tell you a little more about some popular paradigms. Starting with the T-maze.
 English
English German
German French
French Italian
Italian Spanish
Spanish Chinese
Chinese
