How to capture the 3D swimming patterns of fish
Zebrafish have more in common with humans than meets the eye. This is why they have become a “go-to” model in neuroscience research. But one difference remains: we walk and they swim.
Posted by
Published on
Thu 01 Oct. 2015
Topics
| 3D Tracking | EthoVision XT | MediaRecorder | Track3D | Zebrafish |

We have learned that zebrafish have much more in common with humans than meets the eye, which is why they have become a “go-to” model in neuroscience research. But one obvious difference remains: we walk and they swim, which means movement in 3 dimensions. So while video tracking from one camera angle (e.g. above) can give us a lot of information about the movement of humans (or rodents), all the information from the third dimension (depth) is entirely missed from single camera tracking.
3D tracking makes a difference
Researchers from Allan Kalueff’s lab demonstrated four years ago that in some studies, tracking depth can really make a difference. When examining different effects from known anxiolytics and anxiogenics, they found that 3D tracking revealed much more pronounced differences in zebrafish behavior, and that some behavioral effects are even undetected when you limit your observations to 2D video tracking.
So why are we not all tracking in 3D right now? There are, of course, some obstacles: computing power, equipment and space limitations, and more. But, as I already mentioned in this blog post, at Noldus we have been working together with the Kalueff lab to improve a software program called Track3D. This revolutionary program has led to some very promising results, now published in the Journal of Neuroscience Methods.
The humble origins of Track3D
Track3D is not all new; it was, in fact, originally designed to track movement of insects in a wind tunnel. Over the years, researchers found new applications for the software, and have benefitted from updated technologies. Currently, advances in data-processing, high-resolution cameras, calibration methods, and a combination of highly advanced software programs provide a reliable way to track the movement of zebrafish in X,Y, and Z coordinates, as the Kalueff Lab reports in their latest publication (Stewart et al., 2015).
Novel tank testing: validation with Track3D
In this study, 3D tracking results were compared to manual observations of zebrafish behavior in a novel tank test, post application of the psychotropic drugs nicotine, LSD, and PCP. The results showed a strong correlation with the known phenotype of these drugs, the manual method of tracking, and the 3D Tulan methods from previous publications (Cachat et al., 2011 and Cachat et al., 2013). In addition, with 3D tracking the researchers were able to discover behavioral patterns that could be missed with 2D tracking. For example, angular change in 3D (change in velocity vector calculated for each point in the 3D track relative to the previous point), is markedly elevated in PCP- but not in LSD-treated animals. “Wall” swimming at the surface is observed in nicotine-treated zebrafish; both of these behaviors are more easily missed with traditional 2D.
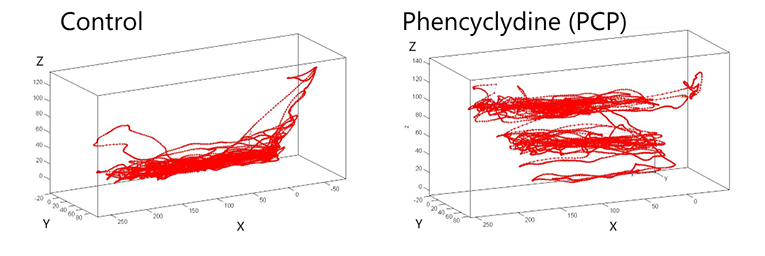
Example of visualization of results when combining data into a 3D trajectory.
Challenges of 3D tracking
Setting up a 3D tracking system does entail adjustments in the experimental set-up. Tracking in 3D requires two synchronized cameras, and the testing arena must be calibrated with real-world dimensions. In this experiment, the first challenge was tackled by Noldus’ MediaRecorder, software that automatically synchronizes multiple video feeds. Calibration took place via use of a specially designed unit with markers, placed in the testing tank; all other calculations occurred within the Track3D software, after video tracking was captured.
Process of 3D tracking
First, both of the two synchronized video feeds were tracked within EthoVision XT video tracking software, as the actual tracking of each video takes place in 2D. The resulting EthoVision XT data output was then combined in Track3D (functioning as an add-on module to EthoVision XT) to get a 3D trace, visualization of this track, and analysis. The combination of two 2D tracks into this 3D visualization, with data points for analysis, allows for the gathering of much more information than from 2D tracks alone, as can be seen in the current methodology article.
To the future
3D tracking holds great promise for the future, particularly given the rising number of zebrafish studies in drug-phenotyping research. If you would like more detail, I recommend reading the publications listed below. If you think 3D tracking might be interesting for your lab, please contact your local sales representative.
Read more
- Soleymani, A.; Cachat, J.; Robinson, K.; Dodge, S.; Kalueff, A.V.; Weibel, R. (2014) Integrating cross-scale analysis in the spatial and temporal domains for classification of behavioral movement. Journal of Spatial Information Science, 8, 1-25.
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.J.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.J.J.; Kalueff, A.V. (2015). A novel 3D method of locomotor analysis in adult zebrafish: implications for automated detection of CNS drug-evoked phenotypes.Journal of Neuroscience Methods, 255, 66-74.
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. (2011). Three-dimensional neurophenotyping of adult zebrafish behavior.PLoS ONE, 6(3), e17597.
- Cachat, J.M. (2013). Developing zebrafish models of complex phenotypes relevant to human brain disorders. Neuroscience, Tulane University: New Orleans, 2013: 150.
Related Posts

Zebrafish research: growing demands in South America