Breathe easy, a novel approach to monitoring respiratory activity in zebrafish
Researchers from the University of Heidelberg used DanioScope in a novel way to objectively measure breathing in zebrafish embryos and larvae.
Posted by
Published on
Tue 30 May. 2023
Topics
| DanioScope | Physiology | Toxicity | Zebrafish |
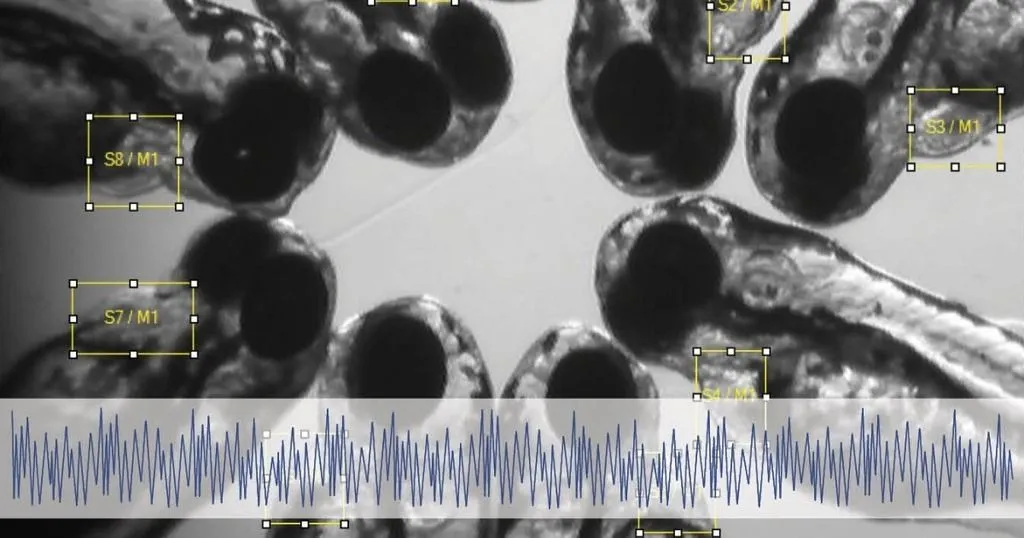
Take a deep breath, nice, isn’t it? Breathing is an essential part of maintaining bodily function for all animals. Therefore, when oxygen homeostasis is disrupted, the body tends to react by adjusting the breathing parameters. However, measuring the breathing movements objectively has been proven difficult in small organisms like zebrafish (Danio rerio) embryo/larvae.
This blog will explore a novel implementation of our product DanioScope, by researchers from the University of Heidelberg. Nadine Kämmer and her colleagues used DanioScope to objectively measure breathing movements of zebrafish and compared this method to a manual assessment [1].
Why not just measure breathing manually?
Maintaining oxygen homeostasis is very important, as oxygen is needed for the aerobic production of energy from oxidative phosphorylation to maintain cell metabolism). When exposed to a low oxygen environment (hypoxia) fish will tend to increase breathing movements in order to enhance oxygen extraction. Contrarily, when exposed to a high oxygen environment (hyperoxia) they will save energy by breathing less, which also prevents excess oxygen uptake and thus, oxidative stress. However, a potential negative side effect of this decreased breathing frequency, is the build-up of CO2 in the bloodstream, causing acidosis.
From this we can conclude that the breathing response is a good indicator of the physiological response of a fish to its environment. However, manually counting breaths in zebrafish is time-consuming and most of all unreliable. Problems with both inter -and intra-reliability play a part in making manual counting unfeasible in the modern age of science.

A lack of good objective protocols
While objective measures do exist, they are not optimal for most studies. Studies have been performed by attaching electrodes to the operculum (series of bones that provide structural support and provide protection to the gills). However, this method is very invasive and only works on larger fish species and not for embryos and larvae.
Electrodes have also been used in a non-invasive way by detecting changes in water conductivity, which is mediated by bioelectrical signals. However, because of the size of fish embryo, their body movements can interfere with the detection of breathing movements and is therefore not suitable for research in fish embryos.
Every breath you take, I will be measuring you
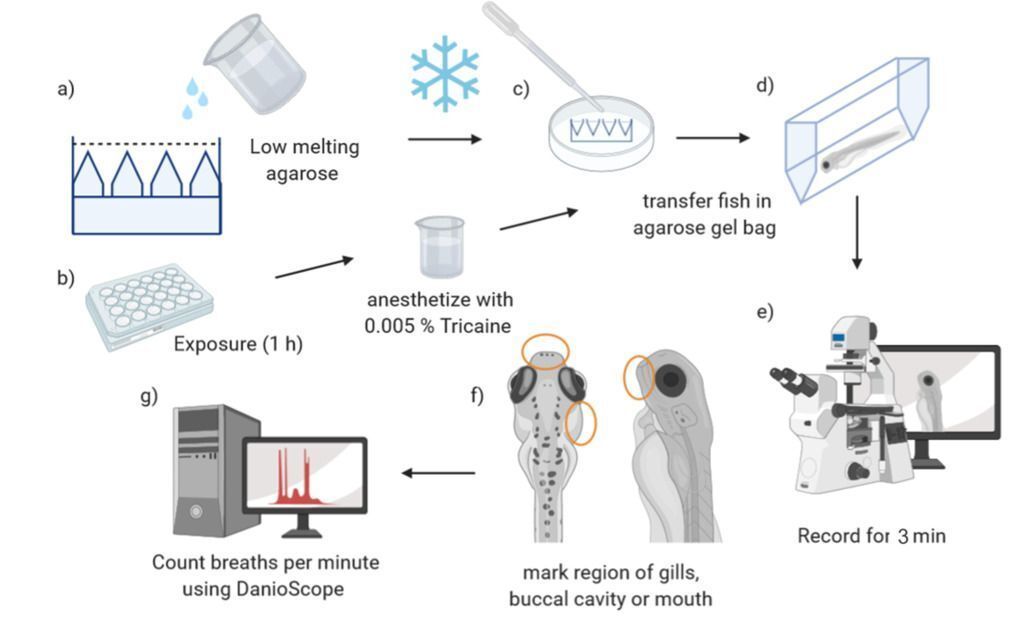
Nadine Kämmer and her colleagues set out to solve this problem. To compare breathing movements and responses in different life stages of zebrafish, measurements were taken in 4 d old zebrafish embryos and in 12 d old larvae. Fish were short-term exposed (1 h) to different concentrations of lindane (neurotoxic pesticide causing respiratory stress), a solvent control (DMSO), or a negative control in water.
Video recording was done while the fish embryos were anesthetized to prevent body movements intervening with the measurements. After transferring the fish to a gel bag, they were acclimatized for 2 minutes, then the fish were video-tracked under a microscope at a 25-fps frame rate.
These videos were then used for both manual counting as well as the analysis in DanioScope. Manual counting was done by two observers by looking at the mouth or the operculum of the fish. Here one breath was defined as the movement of one of these body parts (depending on the orientation of the fish).
A novel use of DanioScope
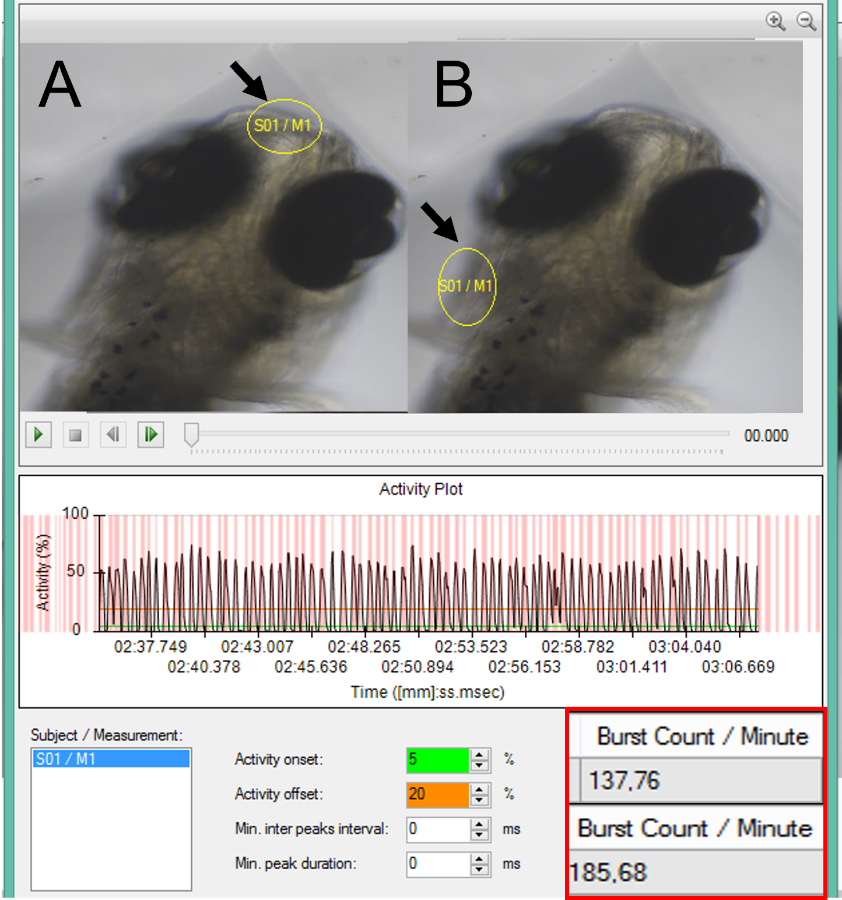
To facilitate the objective measurements Noldus’ DanioScope was used. Originally DanioScope was designed to automatically detect activity in early zebrafish. It can measure many parameters like: Burst activity/ duration, inactivity, but also more specific physiological parameters, like tail coiling and blood/gut flow. It does this analyzing the video for gray scale pixel changes.
Since, DanioScope doesn’t have a build in detection for breathing some pre and post processing steps had to be undertaken by the researchers. In summary, the frame rate of their imported videos had to be made constant, since DanioScope does not work with irregular frame rates. Then, areas of interest (operculum, mouth, or buccal cavity) had to be drawn in the program, after which the detection settings were optimized for this study. For the exact steps undertaken by Kämmer et al. we refer you to their article.
Where do we go now?
In general, measurements with DanioScope revealed a much higher breathing frequency in stressed fish when compared to manual counting. This could mean that manual counting could miss instances of breathing movements, especially in higher lindane concentrations, when the breathing frequency increased dramatically, and movements become fast and irregular. Moreover, manual counting in these situations underestimated breathing frequency by up to 250%.
With this novel application of DanioScope, the researchers were able to successfully measure both breathing frequency and amplitude in zebrafish larvae and embryos. These objective measures can prove very valuable in zebrafish research, especially in toxicology and pharmacology. It enables researchers to see the effects of certain substances and drugs in different life stages, where this was previously not possible.
Kämmer et al. concluded by saying that while some steps are needed to perform this analysis with DanioScope, it is an interesting subject for further research and validation. Furthermore, the manual setting of the regions of interest could also prove useful for detecting other small movements in zebrafish, like eye movement or the passage of plastic particles through the intestine and/or gills.
We would like to thank Nadine Kämmer for providing illustrations and feedback on this post. If you like to learn more about this methodology you can check out Nadine Kämmer’s LinkedIn page or her next paper: Neurotoxic pesticides change respiratory parameters in early gill-breathing, but not in skin-breathing life-stages of zebrafish (Danio rerio), which is currently under preparation for the journal of Aquatic Toxicology.
References
1. Kämmer, N.; Reimann, T.; Ovcharova, V.; Braunbeck, T. A Novel Automated Method for the Simultaneous Detection of Breathing Frequency and Amplitude in Zebrafish (Danio Rerio) Embryos and Larvae. Aquat. Toxicol. 2023, 258, 106493.
Related Posts
Tracking zebrafish in 3D
What can I use DanioVision for?

