
Time based relations in gait analysis
In my last two blog posts, I wrote about static gait parameters. Specifically, what a single footprint can tell you and what kind of information you can get from the distance relationships between prints. Now it’s time to talk about all four paws, and the time based relationships between them. If you ask me, we’ve been saving the best blog post for last!
Temporal relations
Temporal relations are the parameters that have to do with time, such as timing and duration. This is where automation of gait research shows it true colors.
So why would you want to know about the timing of footfalls? Let’s say you have two rats that walk in exactly the same way, with the same distances between each paw print, and the same pattern of walking. But one gets to the other side faster. In theory, this is because one walks faster. In other words, while the distance between the successive placement of one paw remains the same, the time it took to move this foot, was less. The stride (stance plus swing phase of the foot) was faster, not longer. This might be caused by a shorter stance phase, a shorter swing phase, or both, depending on the disorder that underlies this symptom.
Before modern gait analysis systems were used, these temporal differences stayed obscured. Nowadays, systems like CatWalk XT can calculate over 30 different temporal gait parameters.
Temporal parameters in action
Let me give you some examples of these parameters in action:
Shorter stance
If your leg or foot hurts, you don’t want to put your weight on it. This is exactly what animal models of several disorders show. Animals are found to keep their affected paw on the ground for a shorter amount of time in studies on sciatic nerve injury [1,2,3,11], ischemic stroke [7], and arthritis [10]. Additionally, Hoffman et al. [8] saw this compensated in other paws, in study on arthritis.
When the stance duration decreases, the stand duration relative to the total stand and swing duration often also decreases, as Ferland et al. [5] show in their arthritis model. In a study of Encarnacion et al. [4] on ischemic stroke, all paws show shorter time on the ground, but a shorter duty cycle was only found in one paw.
Longer stance
Contrary, in spinal cord injury research [9] the hind paws stay longer in contact with the ground. This is compliant with the typical ‘dragging of the hind body’ seen in spinal cord injury models. This longer stance phase is also found in the Parkinson’s model used by Westin et al. [12].
Longer swing
In most instances, a shorter time on the ground is compensated by a longer air time of that paw, reflected in a longer swing duration. This is found in models of sciatic nerve injury [1,2,3,11] and arthritis [5]. Certain treatments are proved effective in studies on sciatic nerve injury [11] and spinal cord injury [6].
Decreased swing speed
In some instances, a decreased swing speed is measured, meaning that the longer air time does not result in a different stride length. Examples include models of sciatic nerve injury [1] and arthritis [5]. Galvan et al. [6] measured an increase in swing speed after the treatment of spinal cord injury models. In relation to the longer stance phase of their model, Westin et al. [12] also found a decreased swing speed.
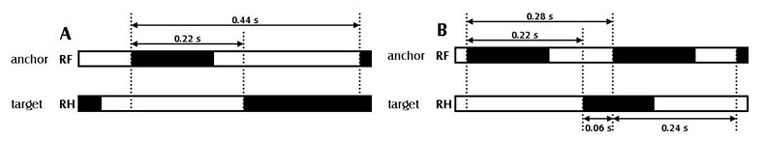
Inter-paw coordination
Inter-paw coordination is reflected in several parameters. For example, phase dispersions and couplings describe the relative placement of two paws, the “target” and the “anchor” paw, during one stride. Kloos et al. [9] found deficits that correlate with the amount of recovery in a spinal cord injury model, while Bozkurt et al. [1] found that this parameters was affected in pairs that included the affected paw, reflecting a delayed positioning of the delayed paw. This was also seen by Deumens et al. [3].
Find out more
I hope you enjoyed our blog series on gait parameters. If you want to learn more, you can always download our free white paper on gait analysis.
Or read more on our website about gait analysis research or automated gait analysis.

Other blogs in this series
Other blogs in this series are now online:
- What gait can tell: 3 blogs that will help you understand
- What a print can tell
- Going the distance - and why it matters in gait analysis
References
- Bozkurt, A.; Deumens, R.; Scheffel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Führman, T.; Brook, G.A.; Pallua, N. (2008). CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. Journal of Neuroscience Methods, 173, 91-98.
- Bozkurt, A.; Scheffel, J.; Brook, G.A.; Joosten, E.A.; Suschek, C.V.; O’Dey, D.M.; Pallua, N.; Deumens, R. (2011). Aspects of static and dynamic motor function in peripheral nerve regeneration: SSI and CatWalk gait analysis. Behavioural Brain Research, 219, 55-62.
- Deumens, R.; Jaken, R.J.P.; Marcus, M.A.E.; Joosten, E.A.J. (2007). The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. Journal of Neuroscience Methods, 164, 120-130.
- Encarnacion, A.; Horie, N.; Keren-Gill, H.; Bliss, T.M.; Steinberg, G.K.; Shamloo, M. (2011). Long-term behavioral assessment of function in an experimental model for ischemic stroke. Journal of Neuroscience Methods, 196, 247-257.
- Ferland, C.E.; Laverty, S.; Beaudry, F.; Vachon, P. (2011). Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacology, Biochemistry and Behavior, 97, 603-610.
- Galvan, M.D.; Luchetti, S.; Burgos, A.M.; Nguyen, H.X.; Hooshmand, M.J.; Hamers, F.P.T.; Anderson, A.J. (2008). Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. The Journal of Neuroscience, 28(51), 13876-13888.
- Hetze, S.; Römer, C.; Teufelhart, C.; Meisel, A.; Engel, O. (2012). Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. Journal of Neuroscience Methods, 206, 7-14.
- Hoffmann, M.H.; Hopf, R.; Niederreiter, B.; Redl, H.; Smolen, J.S.; Steiner, G. (2010). Gait changes precede overt arthritis and strongly correlate with symptoms and histopathological events in pristane-induced arthritis. Arthritis Research & Therapy, 12, R41.
- Kloos, A.D.; Fisher, L.C.; Detloff, M.R.; Hassenzahl, D.L.; Basso, D.M. (2005). Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Experimental Neurology, 191, 251-265.
- Möller, K.Ä.; Berge, O.-G.; Hamers, F.P.T. (2008). Using the CatWalk method to assess weight-baring and pain behaviour in walking rats with ankle joint monoarthritis induces by carrageenan: effects of morphine and rofecoxib. Journal of Neuroscience Methods, 174, 1-9.
- Sheu, M.L.; Cheng, F.-C.; Su, H.-L.; Chen, Y.-J.; Chen, C.-J.; Chiang, C.-M.; Chiu, W.-T.; Sheehan, J.; Pan, H.-C. (2012). Recruitment by SDF-1α of CD34-positive cells involved in sciatic nerve regeneration. Journal of Neurosurgery, 116, 432-444.
- Westin, J.E.; Janssen, M.L.F.; Sager, T.N.; Temel, Y. (2012). Automatic gait analysis in bilateral Parkinsonian rats and the role of L-DOPA therapy. Behavioural Brain Research, 226, 519-528.
Get the latest blog posts delivered to your inbox - every 15th of the month
more

Gait recovery and other effects: treatment of cervical myelopathy
At the Dr. Michael Fehlings' lab, they are on a quest to find out what exactly causes the lower success rate of delayed surgery in cervical myelopathy.
How to really challenge mice cerebellar plasticity
Jan-Willem Potters used the ErasmusLadder in his thesis research to study the role of specific mutations of plasticity in the cerebellar microcircuit of mice.

