Professional software, hardware, and services for your animal behavior research
Automate your research, save time, and get high-quality data for your project or publication. Whether you're in academia, education, or a commercial field, Noldus is here to help you
Schedule a call with an expertHow can we help you advance your research today?

Overview of
software and hardware

Overview of
research applications
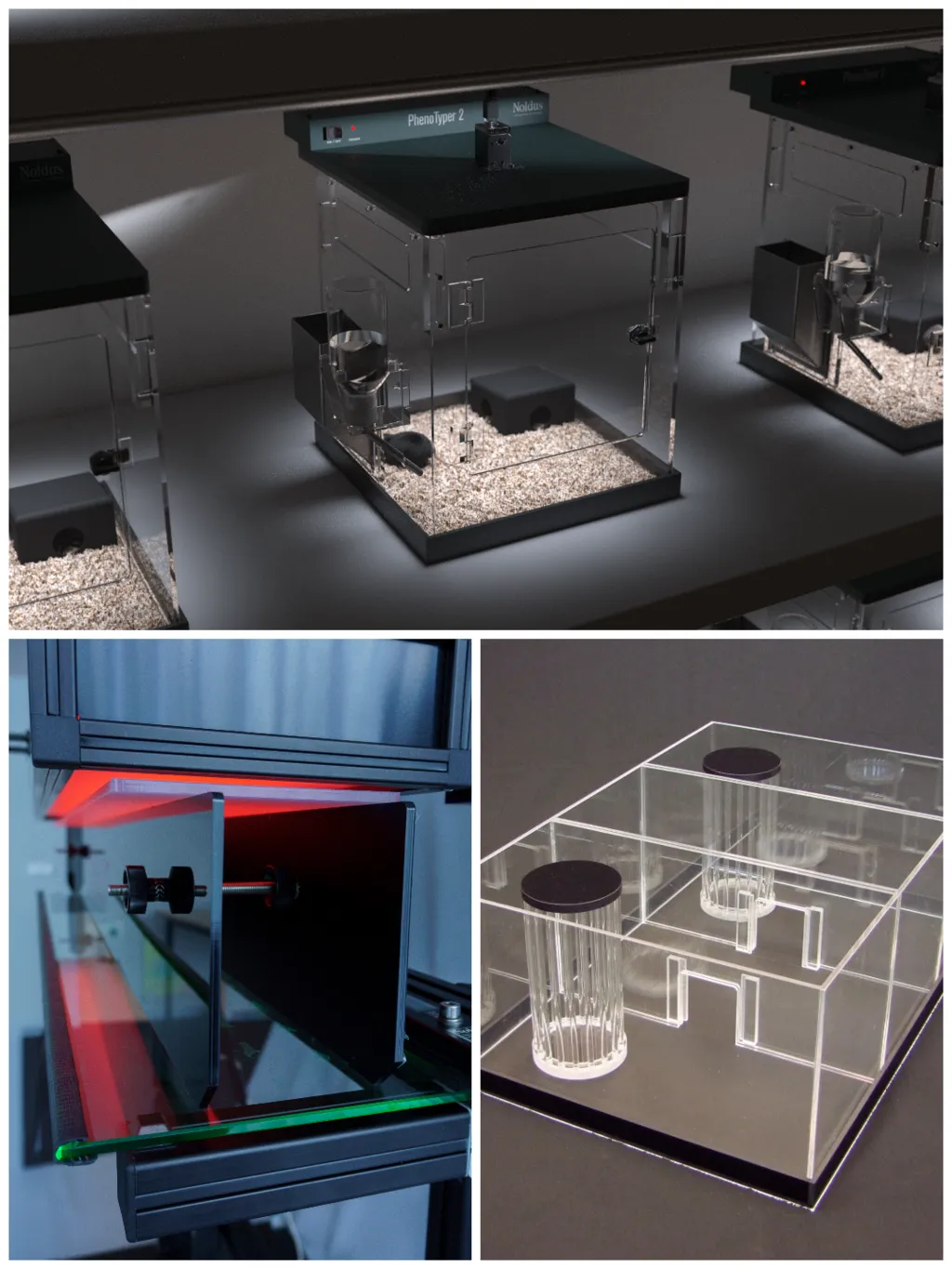
Everything you need for your
research (core) lab
Trusted by researchers around the world






Powerful software, hardware and labs for animal behavior research
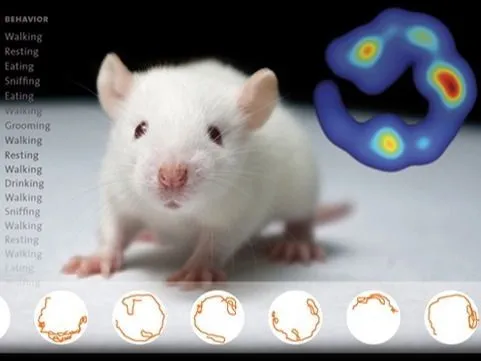
EthoVision XT | Video tracking
Simply the world’s best video tracking software, offering unparalleled precision in tracking animal movement, behavior, and social interaction. Trusted by thousands of researchers, it provides accurate insights and is customizable to meet every research need.

DanioVision | Zebrafish research
The top choice for zebrafish larvae tracking, designed for high-throughput studies and non-invasive observation. Its precision in detecting subtle behavioral changes delivers reliable results, making it ideal for zebrafish research.

PhenoTyper | Instrumented homecage
Ideal for homecage studies in neuroscience research, offering continuous, non-invasive monitoring of rodent behavior in a natural environment, enabling researchers to study complex behaviors over extended periods.

CatWalk XT | Gait analysis
The world’s leading gait analysis tool for neuroscience research, offering precision in analyzing locomotion patterns, helping researchers detect subtle changes in gait dynamics.

Core-lab | Build and integrate
Noldus supports researchers in setting up core labs by providing tailored solutions and guidance, ensuring optimized data collection and enabling efficient, high-quality research for diverse scientific needs.

Mazes and other tools for behavioral neuroscience research
Essential for studying cognition, memory, and spatial learning, mazes and tools like T-mazes or water mazes provide valuable insights into brain function and neurological disorders.
Stay ahead in behavioral research!
Subscribe to Noldus Newsline
- Discover innovations – Be the first to know about new tools and software to advance your research and get insights that help you stay ahead.
- See real research in action – Get inspired by real-world studies from our community, discovering fresh approaches for your work.
- Access exclusive perks – Receive early updates on promotions, product releases, and events crafted for you.
Behavioral neuroscience application areas

Learning and memory
Learning and memory refer to how the brain processes, stores, and retrieves information. Research on these processes helps understand and treat conditions like Alzheimer's, dementia, and amnesia.

Fear, anxiety and stress
These emotional responses are regulated by the brain, and research into these conditions provides insights into therapies for anxiety, depression, PTSD, and related mood disorders.

Long term behavior observation
Continuous monitoring in a homecage environment captures natural behaviors and provides accurate insights into an animal's activity, including feeding, drinking, and sleep cycles.

Social tests
Research on social interactions offers insights into behavior patterns like aggression, cooperation, and bonding, relevant for studying human conditions such as autism and schizophrenia.
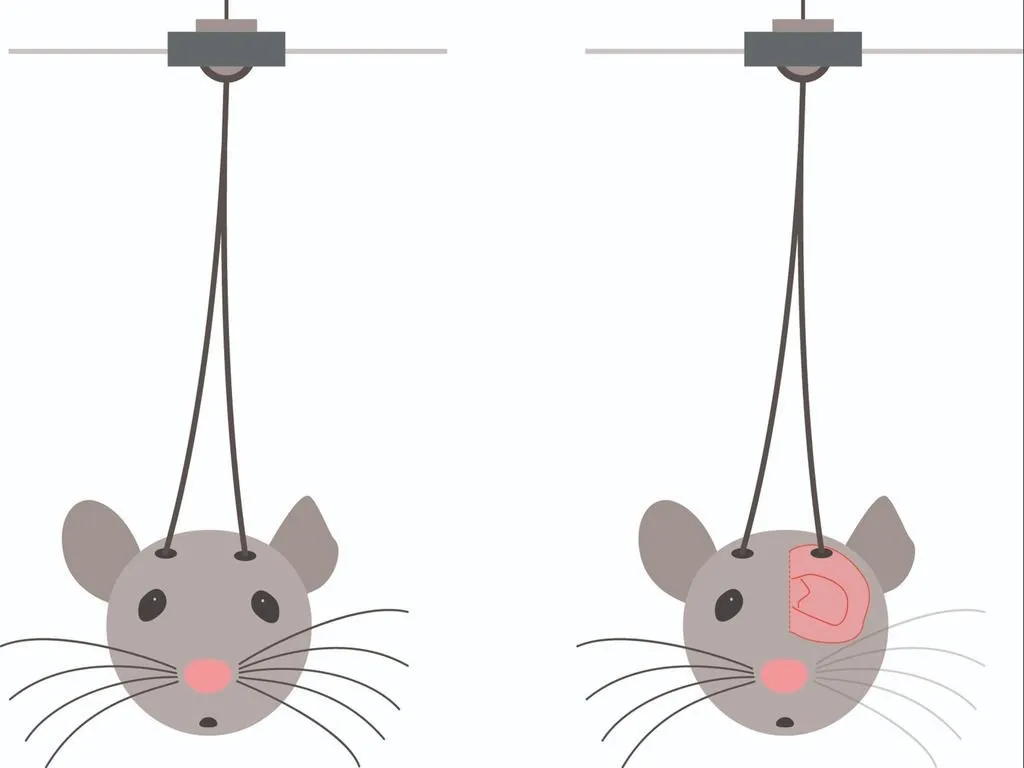
Brain imaging and optogenetics
These techniques allow researchers to control and monitor neuron activity, advancing studies on neurological disorders like Parkinson’s, epilepsy, and depression.

Zoology and ethology
Studying animal behavior has come a long way from manual pen-and-paper methods. Advances in technology now provide faster and more accurate ways to record and analyze behaviors.

Gait analysis
Studies on gait and locomotion assess motor function and the impact of treatments, providing valuable insights into conditions like Parkinson's and spinal cord injuries.
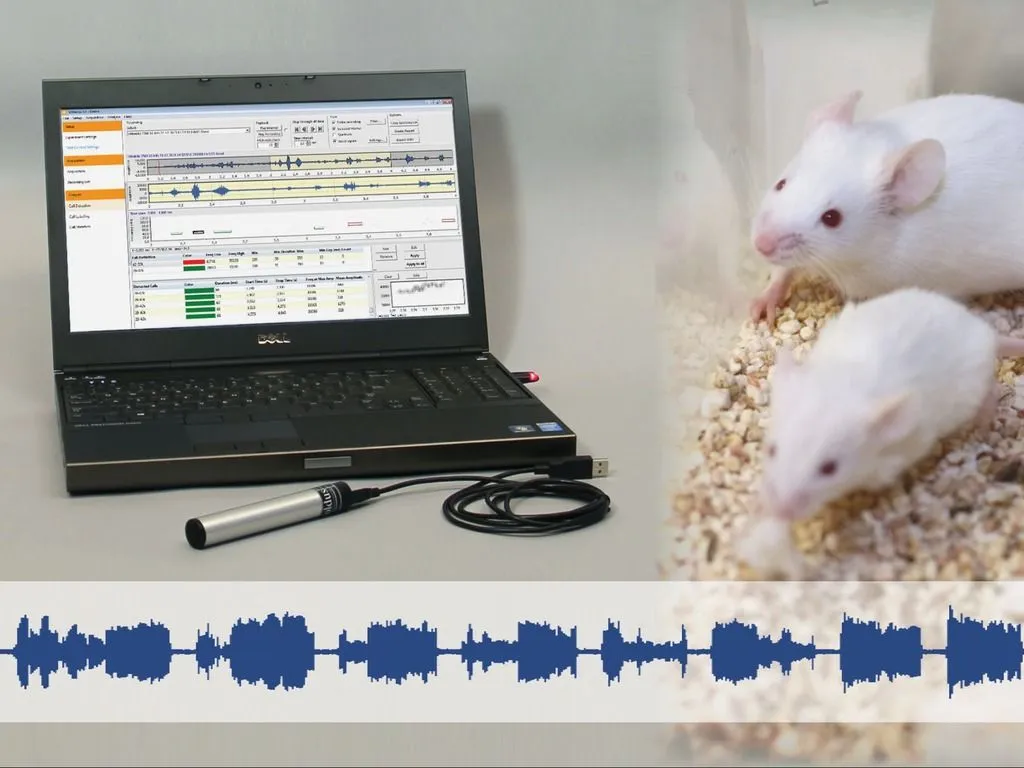
Ultrasonic vocalizations
Analyzing animal vocalizations provides insights into communication, stress, and behavior, enhancing research in neuroscience, pharmacology, and behavioral studies.
Zebrafish research
Zebrafish are used to study treatments for neurodevelopmental disorders and serve as a valuable model for research in ecotoxicology due to their genetic similarities to humans.
Trends & discoveries in animal behavior research
Interactive infographic
Whether you're looking for inspiration or validation for your own research approach, this is a must-see!
Check out how leading researchers use Noldus tools like EthoVision XT, PhenoTyper, and CatWalk XT for deeper insights. This interactive infographic not only reveals key trends, it also let's you explore highlighted publications with a simple click.
Interactive infographic
Whether you're looking for inspiration or validation for your own research approach, this is a must-see!
Check out how leading researchers use Noldus tools like EthoVision XT, PhenoTyper, and CatWalk XT for deeper insights. This interactive infographic not only reveals key trends, it also let's you explore highlighted publications with a simple click.
Everything you need for your (core) lab

Mazes
We offer a wide range of mazes for behavioral neuroscience, perfectly integrated with EthoVision software for high-quality, verifiable data. Whether you need a basic setup or advanced automated mazes, we provide complete solutions and expert training for optimal research outcomes.
Cameras and computers
We work with tested computer hardware and cameras to ensure optimal performance with our software. Many other computers and cameras are also compatible. Visit our webpage for detailed specifications or contact us with any questions.

Expert advice
Creating a dedicated behavioral core lab centralizes testing, expands capacity beyond a single lab, and provides behavioral expertise. This step adds a valuable translational link across scientific fields such as molecular, genetic, and imaging research.

How to build a core lab
This free guide provides comprehensive instructions on building a rodent neurobehavioral core, covering everything from core team setup to equipment planning, logistics, and fee structure, helping optimize research capacity and support training needs.

Behavioral neuroscience tests
This e-book offers an overview of behavioral neuroscience in rodents, including housing, locomotion, anxiety, depression, and cognition tests, with practical protocols, guidelines, and factors that affect rodent behavior and research outcomes.
Buyer's guide to video tracking
This guide helps researchers select the best video tracking setup, covering platforms, cameras, mazes, and software, with an emphasis on accuracy and reproducibility to enhance scientific outcomes.
Don't know where to start?
We're happy to help
Book a meeting for an online or onsite session.
Together, we can discuss your research requirements and come up with the best solution!