Tools for studies with the
Morris water maze
The Morris water maze task is a popular and well-validated test for spatial learning, and is one of the most-used behavioral tests in neuroscience research with rat and mouse models.
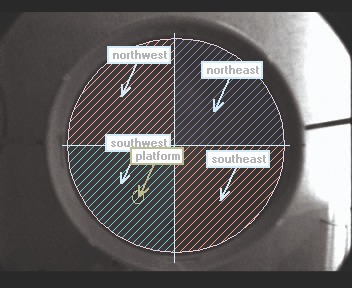
The testing area is a round pool filled with water and a hidden platform is submerged just below the water surface. The rat or mouse learns to escape from the water by locating the platform, in most cases with the help of visual cues. Alternatively, the platform can be placed in another quadrant, or removed during another phase of the experiment. This way, memory retention and extinction can be investigated.

High quality pools
At Noldus, we offer several options for high quality mazes. They are either custom-built by us or one of our trusted suppliers. Several size and color options are available and each maze is from top materials, durable, and easy to clean. Most importantly, these water pools are perfectly suited for video tracking experiments and automated tests. We also supply accessory automated platforms.

Package deal
All water mazes are available in a cost-efficient package deal that includes a computer and a full EthoVision XT software license for video tracking. This license can also be used for video tracking and automation of other behavioral tests.
Video tracking
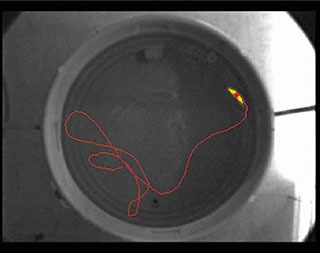
In most water maze trials, the main goal is to measure how quickly the animal locates the platform, and how this ability improves over time. As a result, latency is of importance, but so is the average distance to the platform over time (e.g. Gallagher’s proximity).
The swimming pattern itself can also be of interest. For example, it can be studied in order to uncover the navigational strategy of the animal. Whishaw’s error or heading angle error are examples of parameters that reflect the subject’s strategy.
The location of the animal relative to its previous location is important, especially in trials in which the platform is removed (or lowered so the animal can’t reach it). Therefore, the distance to this location or the time spent in the correct quadrant are important parameters.
The good news is that these parameters are easily measured by video tracking with EthoVision XT. In addition, EthoVision XT offers great data selection and analysis tools, and it has intuitive visualization options.

"Keep the good work going. You make our lives easy and each new version of EthoVision is testimony to the fact that the company is frankly put, the best at its game in the world. We appreciate this very much!"
Dr. De Wet Wolmarans|North-West University, South Africa
Automation options
We have automation options available if you want to use automatica/hydraulic Atlantis platforms. For example, we supply a combination of four platforms, one in each quadrant of the maze, which allows you to easily change the location of the platform over several trials without having to get into the water.
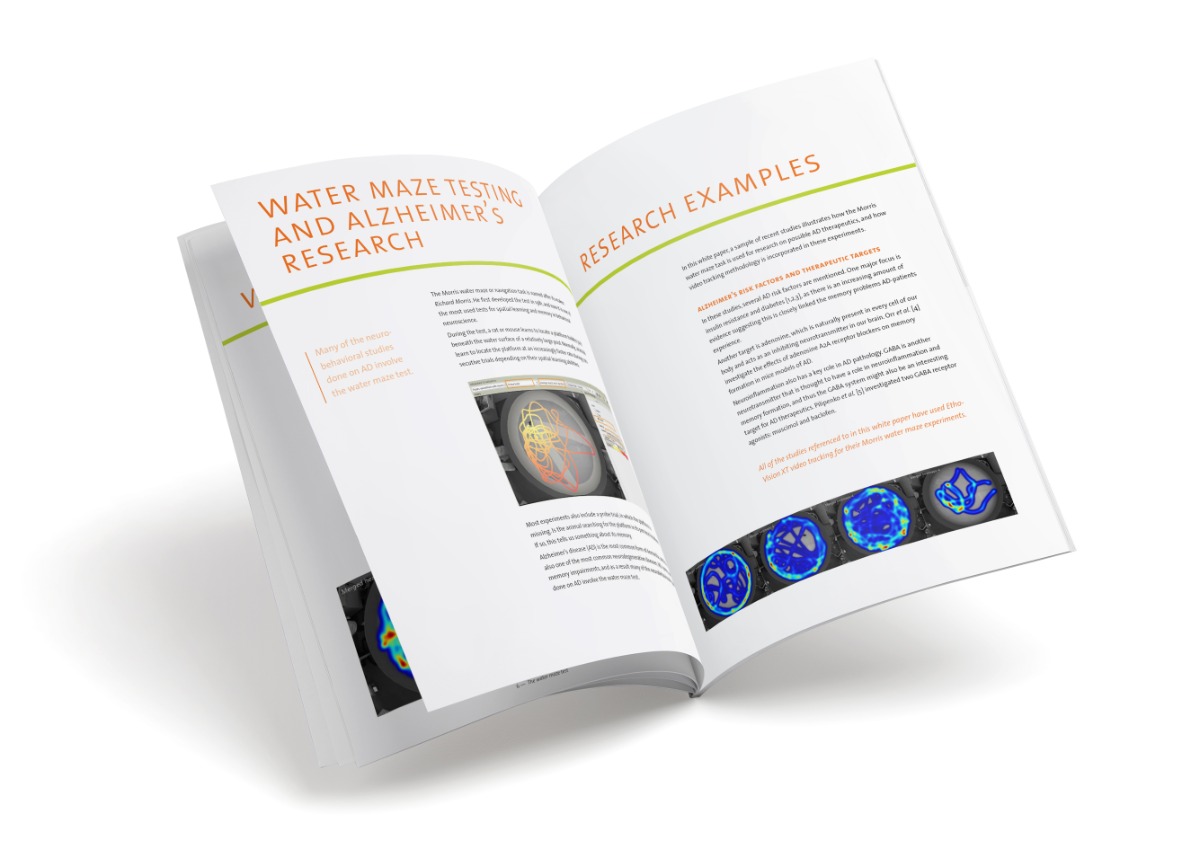
Free white paper
Water maze testing and Alzheimer’s
In this white paper, a sample of recent studies illustrates how the Morris water maze task is used for research on possible AD therapeutics, and how video tracking methodology is incorporated in these experiments.
The Morris Water maze test is one of the most used tests for spatial learning and memory in behavioral neuroscience. Read about how EthoVision XT is ideal for this test!
Dr. Do Rego: "Automatic measurements makes us gain in quality."
Dr. Jean-Claude do Rego and Dr. Jean-Luc do Rego of the Behavioral Analysis Facility of the University of Rouen (Service Commun d’Analyse Comportementale, SCAC), are evaluating the behavioral and functional activities of new pharmacological drugs.
Free e-book
Basic behavioral neuroscience in rodents
Measuring spatial learning and memory in the Morris water maze. Why is this important? And what do we actually measure in this neurocognitive test? Our free e-book explains the basic behavioral neuroscience behind the Morris water maze and other tests, and how they are used.
Learn more
- Find out more about EthoVision XT tracking software,
- Or read more about The Morris water maze on our Noldus blog.
Relevant blogs

Connects some dots - cognitive impairment and cranial radiation
On a yearly basis, an estimated 20.000 individuals are diagnosed with primary brain tumors in the United States alone. About ten times that number of patients will receive treatment for primary or metastatic brain cancer.
EthoVision XT and the Morris Water Maze: expert tips and tricks
Neuroscientist Colleen McSweeney, Ph.D. shares her expert knowledge on using EthoVision XT and the Morris Water Maze. From a brief history to valuable tips and tricks, here is all you need to know on automated tracking.
 English
English German
German French
French Italian
Italian Spanish
Spanish Chinese
Chinese