EthoVision XT
A platform for video tracking
EthoVision XT has a flexible, modular approach - a platform to build on for the future. Configurations range from basic video tracking to a multi-functional (automated) system. On this page we explain different modules. Of course we are always happy to help you decide what suits your experiments best!
Modular approach
EthoVision XT starts with the base version. This is meant to be used to track the center point of 1 subject in 1 arena. Additional modules can be added on depending on your experimental needs and budget.
EthoVision XT is the most widely applied video tracking software that tracks and analyzes the behavior, movement, and activity of any animal.
-
A cost-effective solution for all standard behavioral tests such as the Morris water maze and open field testing
-
High-throughput and high-content testing
-
Suitable for sophisticated test-protocols

“What I love about Noldus Information Technology is the personal attention and service. I am not dealing with a website or a call center in a remote country but with people whom I know by name, and who provide advice and on-site support during periodic visits.”
Prof. Dr. Daniela Pollak|Medical University of Vienna, Austria
EthoVision XT Base
The Base version of EthoVision XT gives you all you need to track and analyze the movement, activity, and behavior of one animal in one arena at a time. It guides you through all phases of your experiment, from setting up your project to detailed data analysis. You can perform a wide range of experiments, including common applications like the open field, the Morris water maze, and an elevated plus maze.
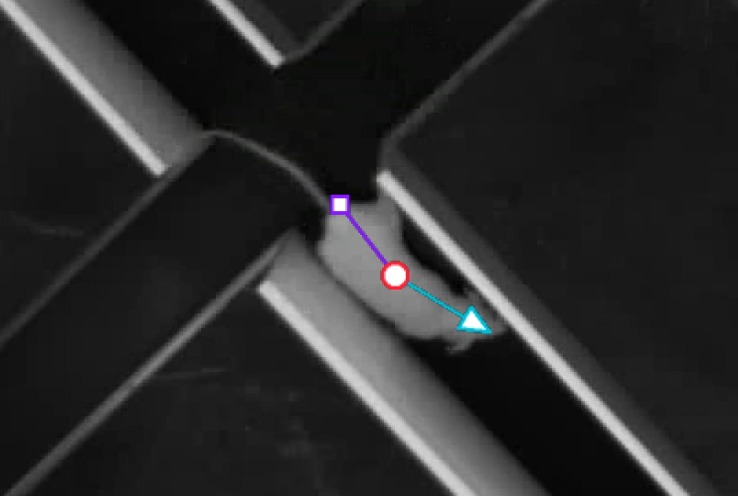
Base includes the option to track the nose-point and tail-base in addition to the center point of your rats, mice, or zebrafish. Multiple body points tracking is designed for top-view tracking.
Tracking multiple body points gives you added information about the proximity of the animal's nose to a certain object (indicating interest of novelty), or about the direction the animal's head is pointed towards. It also increases the accurately when analyzing entrances into certain areas, such as the arms of an elevated plus maze.
- Reliably detect and track your animal even when there is low contrast.
- Combine any type of arena, enclosure, or maze.
- Acquire data using a live video feed or from prerecorded video files.
- Tracking the nose point, center point, and tail base.

Track in multiple arenas
Multiple Arena Module
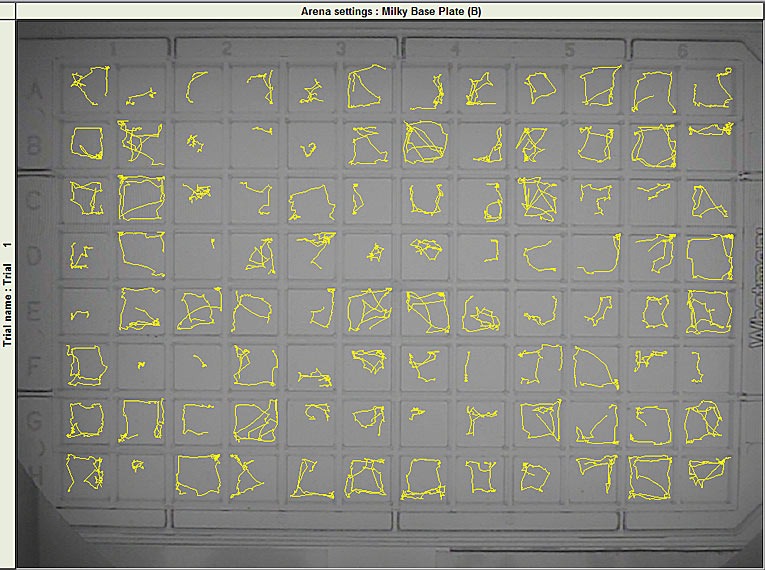
While most systems cannot track in more than eight arenas, EthoVision XT’s Multiple Arenas Module tracks animals in up to one hundred arenas simultaneously.
Increase your throughput by analyzing animal behavior in four, eight, or even sixteen open fields at the same time, or track small organisms in multi-well plates. You can define start and stop conditions for a trial to be applied to each arena individually.

Study social behavior
Social Interaction Module
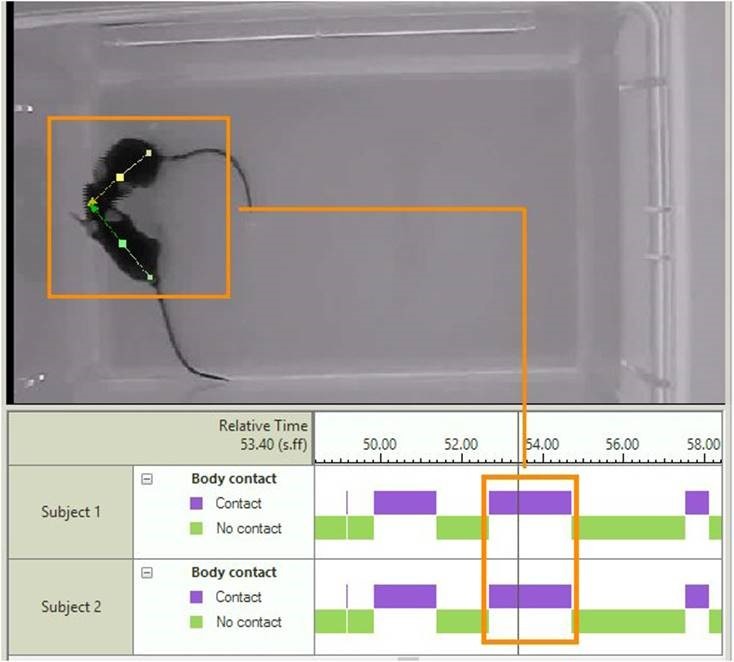
Often used in studies on anxiety, aggression, schizophrenia, and other psychiatric disorders, the Social Interaction Module allows you to track multiple animals per arena simultaneously.
Social parameters include proximity and the amount of time an animal spent moving away from another animal. In addition, you can measure body contact based on the position of body contours instead of body points.

Automatic behavior recognition
Behavior Recognition Module
Traditionally, human observers are trained to recognize rat behaviors. This takes time, is prone to repeatability issues, and is inherently subjective. The Rat Behavior Recognition Module and the Mouse Behavior Recognition Module are objective, accurate, and tireless.

Integrate external data
External Data Module
Physiological data, such as heart rate, blood pressure, and neuronal activity, and other external data, like vocalizations and environmental temperature or humidity, can all add valuable information complementary to your behavioral data.
The External Data Module integrates all these data, so you can visualize, select, and analyze all your data in sync.


Control external equipment
Trial & Hardware Control Module
Complete automation of research saves time, effort, and money. Not only does it make things easier, more importantly it enhances the reliability and validity of your results. This is what the Trial & Hardware Control Module offers: it lets you control external equipment from within EthoVision XT and define experiment protocols.

Quality Assurance
Quality Assurance Module
The Quality Assurance protects your data and creates log files of everything that goes on during a project. It has been developed with laboratory researchers and technicians from large pharmaceutical companies to comply with quality assurance protocols and guidelines.
You can assign different rights to different users of EthoVision XT, and this module is compliant with 21 CFR Part 11 (see FDA), the regulations on electronic records and electronic signatures for studies conducted under Good Laboratory Practice (GLP).
 English
English German
German French
French Italian
Italian Spanish
Spanish Chinese
Chinese